Improving your website’s user experience – AndroGel example
 Many factors influence your user’s experience when visiting your website or reading your blog (functionality, content, usability, look and feel – the list goes on). Ultimately, you have to remember that the “user” is a person and think about how your website (often their first interaction with your firm), will affect their understanding of your ability to help them. In part one of this two-part series, I’m going to talk about blog and website content, the importance of freshness, and what makes copy engaging and interesting (all in the interest of improving your client’s user experience).
Many factors influence your user’s experience when visiting your website or reading your blog (functionality, content, usability, look and feel – the list goes on). Ultimately, you have to remember that the “user” is a person and think about how your website (often their first interaction with your firm), will affect their understanding of your ability to help them. In part one of this two-part series, I’m going to talk about blog and website content, the importance of freshness, and what makes copy engaging and interesting (all in the interest of improving your client’s user experience).
I wanted to use a real-life example that would reflect a relevant current event and show you the wide variety of results. I searched the term “AndroGel class action” and reviewed only the first page results. Specifically, I looked at each landing page to find anything about the low-testosterone replacement drug AndroGel, and then went to the blog for each site looking for AndroGel news updates. If a firm or lawyer appeared two or more times in the results, I only used one of the results, and I only reviewed the sites of firms or attorneys, not marketing or information sites. I’m discussing the results in the order of my article, not in the order they appeared in the results.
The firms included in this review are-
- Morgan & Morgan (http://www.forthepeople.com/class-action-lawyers/testosterone-therapy-lawsuit)
- The Schmidt Firm, LLP (http://www.schmidtlaw.com/androgel-class-action-lawsuit/)
- Schmidt & Clark, LLP (http://www.schmidtandclark.com/androgel-class-action-lawsuit)
- Saiontz & Kirk (http://www.youhavealawyer.com/androgel/)
- Seeger Weiss, LLP (http://www.drugdangers.com/testosterone/androgel)
Update Frequency
There are no hard and fast rules about how often you should update a blog or website. That said, what’s most important is that your site content and the posts on your blog reflect current topics and ideas. In early March, the U.S. Food and Drug Administration updated the warning label for testosterone therapy drugs. This was pretty big news for those involved or interested in a class action against the drug makers. I would expect that each firm would have information on the new warning and I’m hoping to find blog posts from each firm detailing what the new warning means as well as any other new and related information.
Morgan & Morgan included an update on the page I clicked to from the results (see below).

Saiontz & Kirk, P.A., Seeger Weiss, LLP, Schmidt & Clark, LLP and Schmidt Law didn’t include a reference to the FDA updated warning label on the pages I landed on. In fact, the last viewable update on the Schmidt Law page is from July 2014. If I was deciding on a firm to file my lawsuit, I don’t know how comfortable I’d be with a firm that doesn’t appear to be on top of the latest news or sharing information with potential clients who visit their website (most likely from a search similar to the one I did).
Blog Content and the FDA Update
There’s no clearly labeled blog section for The Schmidt Firm, LLP and the “Related Post” section at the bottom of the page doesn’t list an FDA update post. Schmidt & Clark, LLP also doesn’t have a blog, latest news, or learn more section on the page that I landed on, leaving me unable to determine if they’re even aware of the FDA warning. Again, this surprised me and is detrimental to the user experience in this case.
Morgan & Morgan, Saiontz & Kirk, PA, and Seeger Weiss LLP all have posts on the latest FDA warning in their “Blog,” “Learn More,” and “Latest News” sections, respectively. Each firm discusses the new warning, why the government agency is requiring the new label, as well as the standard history of the allegations and how they can help you file a lawsuit.
Creating Engaging and Interesting Copy
If we look at the blog posts provided by the firms that reported on the FDA update, we see that the three firms approached the update differently.
Morgan & Morgan’s post explains the FDA update, how the drug will be used going forward, and warning signs you should consider if you’re taking the drug. The firm maintains a neutral voice and adds little marketing content.
Saiontz & Kirk, PA also explain the FDA warning and they provide some history, a marketing push, and information about filing a lawsuit. Seeger Weiss LLP includes an attorney quote in their post and then explains the controversy surrounding the drug.
All three of these firms fail to make the FDA update “theirs.” With or without the marketing spiel, all three firms simply report the same information, without finding a way to pull the reader in. While an FDA warning update is news itself, all three firms could have tied the update together with something that would have made their posts stand out. Each firm mentions a new study, recent studies, previous studies, a number of studies, or recent data, as is common with defective drugs, but we never really get to dig into the studies. Remember, I’m writing about only the two pages from each firm that I evaluated based on the idea that a real user would click into the site from the search results, and then go to the blog for more information about the update, before going back to review the next search result.
One firm that didn’t rank in the first (or second or third) page of my results reported the update but tied it to the fact that the results of the two studies will be presented this month (the same month as the FDA update). That firm also reported that the results of the studies conflict. Just by giving that additional information (which very much piqued my interest), the lower ranking firm outperformed the others in my personal user experience.
Any firm can regurgitate information, but to enhance user experience, you should make your blog posts stand out and keep your website updated with pertinent information. There are many law firms representing people from across the nation for allegedly defective drugs and each one should be unique in how they reach out to their users.
Each post should accurately represent the firm and reflect the personality and culture of the firm. Imagine how you would describe the FDA update to a family member and try to incorporate that tone and some of those same word choices into your content. Make your content personal and professional. Let it reflect who you are so your users can get to know you. There’s only so many ways to explain an FDA update, make sure that the way you chose to explain information sets you apart.
For firms representing only local clients, it’s essential that you also stand out. You have the advantage of adding in content specifically of interest to your community. You should absolutely connect with your local users by including relevant local information.
Beyond news updates, if your firm sponsors a local event, write up a blog post about the event and the people involved. If your attorneys volunteer with non-profit organizations, write an informational piece about the organization or agency and the positive impact said organization is making in the community.
Balancing Content Mediums (words, videos, images)
Not many people have time to read long detailed blog posts, and not many users want to scroll down long-winded web pages. Content should be broken up with images and videos. Remember, though, that meaningless images do little to help your user experience. Make sure the images convey a meaning that’s clear and distinctly relevant to your post.
Videos can be embedded in your webpage or blog easily and in a variety of sizes. As you see below, Saiontz & Kirk use colors, images, and videos to break up text, make the web page more appealing, and to convey the maximum amount of information. The video is a short infomercial that’s posted to YouTube.com and then embedded to the website.
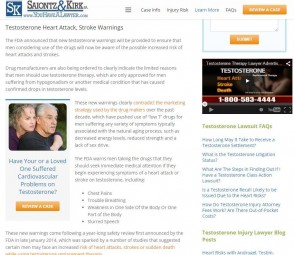
The Saiontz & Kirk FDA update write-up is actually pretty long and the firm does a great job of breaking up the content making it easier to read with adequate spacing, font size, and colors.
In part 2 of this series, I’ll cover how to revamp your attorney profile and write a knock-out bio.
Teresa Shaw is an SEO consultant with 10 years of experience helping attorneys and law firms across the country increase public awareness of their services.

Comments
Everything you pointed out about website designs is accurate, but the average “layperson” consumer isn’t going to know the difference between an official FDA update, and the marketing content of each firm’s blog. Most of these visitors are going to base the trust they put in each attorney website based on things completely unrelated to how consistent information like the FDA warning is presented.
Leave a Reply